|
Experiment No. 4 Conductive Liquids
Experiment: To discover what substance makes the best electrolyte. Materials:
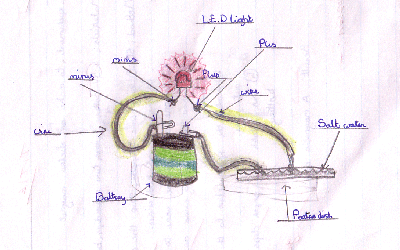
Method: 1) Twist one end of a wire around each paper clip. 2) Hook one paper clip over each terminal of the battery. 3) Twist the
wire that is connected to the + terminal of the battery (a) Tap Water 5) Twist a third
wire around the minus wire of the LED. 6) Place the
loose ends of the wires in each plastic petrie dish in turn. Result: When
you place the wires in the different substances, the LED lights up
(or does not). If you leave Conclusion: We
concluded that Salty Water conducted electricity the best and therefore
made the To discover why
salt has this effect on water, please click
here. Our
Experiments 1. Sticky Balloons | 2.A Simple Circuit | 3. Electro-Magnetism! | 4. Conductive Liquids | 5. Hydrolysis 6. Hydrolysis II | 7. (The Nervous System) Finding your Blindspot | 8. (The Nervous System) One Point, or Two!
| |